Re-designing the substrate binding pocket of laccase for enhanced oxidation of sinapic acid†
Abstract
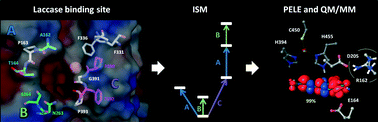
Iterative saturation mutagenesis was performed over six residues delimiting the substrate binding pocket of a high redox potential chimeric laccase with the aim of enhancing its activity over sinapic acid, a lignin-related phenol of industrial interest. In total, more than 15 000 clones were screened and two selected variants, together with the parent-type laccase, were purified and characterized. The new variants presented shifted pH activity profiles and enhanced turnover rates on sinapic acid and its methyl ester, whereas the oxidation of related phenols was not significantly enhanced. Neither the enzyme's redox potential nor the mechanism of the reaction was affected. Quantum mechanics and molecular dynamics calculations were done to rationalize the effect of the selected mutations, revealing the critical role of the residues of the enzyme pocket to provide the precise binding of the substrate that enables an efficient electron transfer to the T1 copper. The results presented highlight the power of combining directed evolution and computational approaches to give novel solutions in enzyme engineering and to understand the mechanistic reasons behind them, offering new insights for further rational design towards specific targets.

 Please wait while we load your content...
Please wait while we load your content...