A Mn(iii) polyoxotungstate in the oxidation of organosulfur compounds by H2O2 at room temperature: an environmentally safe catalytic approach
Abstract
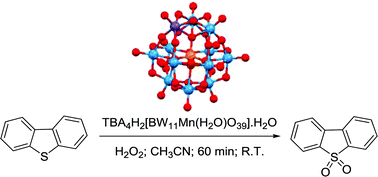
The tetrabutylammonium (TBA) salt of a Keggin-type polyoxometalate (POM), with the chemical formula TBA4H2[BW11Mn(H2O)O39]·H2O, TBABW11Mn, was evaluated as a catalyst in the oxidation by hydrogen peroxide of several organosulfur compounds, namely benzothiophene (BT), 2-methylbenzothiophene (2-MBT), 3-methylbenzothiophene (3-MBT), dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT) and 4,6-diethyldibenzothiophene (4,6-DEDBT), in acetonitrile at room temperature. All compounds were oxidized to their corresponding sulfones with high conversion and selectivity. Following the excellent results achieved, the BW11Mn/H2O2 in CH3CN system was tested in the oxidation of model fuels (MFs) consisting of a mixture of BTs and DBTs in hexane (MF1 containing mainly BTs and MF2 containing predominantly DBTs). In both cases, the organosulfur compounds from the model fuels were fully converted into their corresponding sulfones. Envisaging the development of a promising desulfurization procedure, the extraction of sulfur compounds from MF2 was attempted after the catalytic oxidation process. More than 98 mol% was removed using an ethanol/H2O mixture.

 Please wait while we load your content...
Please wait while we load your content...