Synthesis of α-aminoboronic acids
Abstract
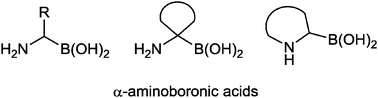
This review describes available methods for the preparation of α-aminoboronic acids in their racemic or in their enantiopure form. Both, highly stereoselective syntheses and asymmetric procedures leading to the stereocontrolled generation of α-aminoboronic acid derivatives are included. The preparation of acyclic, carbocyclic and azacyclic α-aminoboronic acid derivatives is covered. Within each section, the different synthetic approaches have been classified according to the key bond which is formed to complete the α-aminoboronic acid skeleton.

 Please wait while we load your content...
Please wait while we load your content...