Fast and slow dynamics and the local structure of liquid and supercooled water next to a hydrophobic amino acid†
Abstract
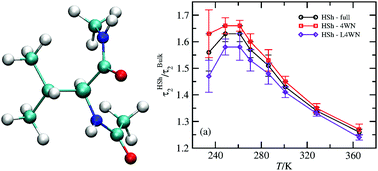
We study, through molecular dynamics simulations, the structure and orientational dynamics of water next to a blocked hydrophobic amino acid, valine (Val), above and below the freezing point of water. The structure and the orientational dynamics of waters with four water neighbors (4WN) and less than four water neighbors (L4WN) in the Val's coordination sphere are deconvoluted. We find that in spite of the excluded volume effects waters with L4WN have faster librational dynamics than bulk water, reminiscent of water at the liquid–vapor interface, and faster orientational dynamics than waters with 4WN, at every temperature. Furthermore, our results show that the pronounced decrease of the orientational retardation factor below ∼255 K observed experimentally is mostly caused by the acceleration of the orientational dynamics of waters with L4WN, while waters with 4WN exhibit only moderate acceleration. The differences between the hydrogen-bond acceptor switching mechanism in the shell and the bulk are also analyzed, and no evidence of especially slow OH groups neither in the 4WN nor in the L4WN populations is found. Finally, we show that waters with 4WN have higher tetrahedrality than bulk water at every temperature although this difference decreases at both high and low temperatures.

 Please wait while we load your content...
Please wait while we load your content...