New insights into UTSA-16†
Abstract
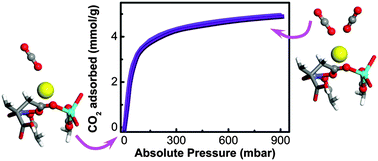
Among the metal organic framework materials proposed for CO2 separation, UTSA-16 possesses the highest CO2 volumetric density explained on the basis of favourable interactions between CO2 and structural water molecules in the material, as revealed by neutron diffraction. In this study, UTSA-16 was synthesised and extensively characterised by XRD, TEM combined with EDX analysis and DR-UV-Vis, Raman and FTIR spectroscopies, as well as by TGA measurements. The synthesised material shows XRD patterns, surface area, CO2 capacity and isosteric heat coincident to the ones reported for UTSA-16 in the original papers but a higher thermal stability and a complete removal of water upon activation under mild conditions (363 K). On the basis of EDX and IR measurements, the formula of UTSA-16 used in the present study is proposed to be K2Co3(cit)2. Infrared spectroscopy clearly shows that UTSA-16 described in this work reversibly interacts with water vapor, CO and CO2. The interaction is attributed to K+ species, which are present as counterions in the pores. At 1 bar and 298 K a fraction of K+ sites adsorbs 2 CO2 molecules.

 Please wait while we load your content...
Please wait while we load your content...