Influence of sumanene modifications with boron and nitrogen atoms to its hydrogen adsorption properties†
Abstract
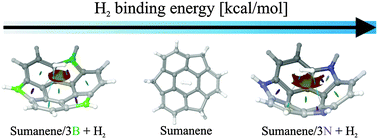
We investigate the influence of sumanene modifications on its adsorption properties towards the hydrogen molecule. The benzylic positions of sumanene were substituted with boron and nitrogen atoms, which changed its hydrogen storage properties. H2 binding energies were calculated using the LMP2, DFT and DFT-D3 approaches with several exchange–correlation functionals and the results indicate a physisorption mechanism. Physisorption was confirmed by fragment analysis and special attention was paid to non-covalent interactions. All non-covalent interactions, based on reduced density gradient surfaces, were identified and calculated for better understanding of the adsorption mechanism. Moreover, the significance of charge separation by inducing boron and nitrogen atoms is emphasized and special attention is paid to the z-component of the dipole moment of sumanene derivatives.

 Please wait while we load your content...
Please wait while we load your content...