Energetics and structural characterization of the “DFG-flip” conformational transition of B-RAF kinase: a SITS molecular dynamics study†
Abstract
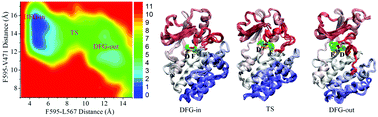
B-RAF protein kinase is a promising target to treat malignant melanoma. The kinase activity of B-RAF is regulated by a “DFG-flip” conformational transition between functional DFG-in and DFG-out states. The difficulty in resolving the activation loop in crystal structures and the even greater difficulty in experimentally capturing high-energy-level transient structures render elusive the molecular mechanism of the B-RAF functional conformational transition. Here, a homology modeling technique and an enhanced sampling molecular dynamics simulation were used to identify and energetically characterize the conformational transition pathway of B-RAF on a multi-dimensional free-energy landscape. The results reveal that the conformational transition is a two-state transition, with the evaluated free-energy barrier comparable to those of other kinds of kinases as reported in the previous literature. Hydrophobic interactions between activation loop and neighboring segments are suggested to dominate the conformational transition and determine the free-energy barrier. The detailed analysis of hydrophobic interactions involved in the conformational transition may show a suitable pathway for the development of the B-RAF inhibitor.


 Please wait while we load your content...
Please wait while we load your content...