Precise determination of water exchanges on a mineral surface†
Abstract
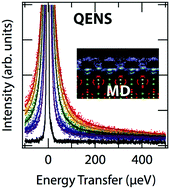
Solvent exchanges on solid surfaces and dissolved ions are a fundamental property important for understanding chemical reactions, but the rates of fast exchanges are poorly constrained. We probed the diffusional motions of water adsorbed onto nanoparticles of the mineral barite (BaSO4) using quasi-elastic neutron scattering (QENS) and classical molecular dynamics (MD) to reveal the complex dynamics of water exchange along mineral surfaces. QENS data as a function of temperature and momentum transfer (Q) were fit using scattering functions derived from MD trajectories. The simulations reproduce the dynamics measured in the experiments at ambient temperatures, but as temperature is lowered the simulations overestimate slower motions. Decomposition of the MD-computed QENS intensity into contributions from adsorbed and unbound water shows that the majority of the signal arises from adsorbed species, although the dynamics of unbound water cannot be dismissed. The mean residence times of water on each of the four surface sites present on the barite {001} were calculated using MD: at room temperature the low barium site is 194 ps, whereas the high barium site contains two distributions of motions at 84 and 2.5 ps. These contrast to 13 ps residence time on both sulfate sites, with an additional surface diffusion exchange of 66 ps. Surface exchanges are similar to those of the aqueous ions calculated using the same force field: Baaq2+ is 208 ps and SO4aq2− is 5.8 ps. This work demonstrates how MD can be a reliable method to deconvolute solvent exchange reactions when quantitatively validated by QENS measurements.


 Please wait while we load your content...
Please wait while we load your content...