Elucidation of the local dynamics of domain-III of human serum albumin over the ps–μs time regime using a new fluorescent label†
Abstract
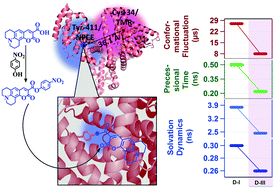
The ps–μs dynamics of domain-III of human serum albumin (HSA) has been investigated using a new fluorescent marker selectively labeled to the Tyr-411 residue. The location of the marker has been confirmed using Förster resonance energy transfer (FRET) study. Steady state, time-resolved and single molecular level fluorescence techniques have been employed to understand the dynamics within the domain-III of HSA. It is found that solvent reorganization dynamics in domain-III is 1.7 times faster than that in domain-I. The timescale of the local rotational dynamics of domain-III is found to be 2.3 times faster than that of domain-I. Fluorescence correlation spectroscopic experiments reveal that domain-III of HSA has more conformational flexibility than domain-I. Together, the results deliver useful details of the local environment around the domain-III of HSA, which have not been explored earlier, mainly because of a lack of a suitable fluorescent marker for domain-III. The newly synthesized probe serves well as a site specific fluorescent marker for HSA, and can be used for further investigation of the ligand binding properties and enzymatic activity of domain-III of HSA.

 Please wait while we load your content...
Please wait while we load your content...