Probing the microhydration of metal carbonyls: a photoelectron velocity-map imaging spectroscopic and theoretical study of Ni(CO)3(H2O)n−†
Abstract
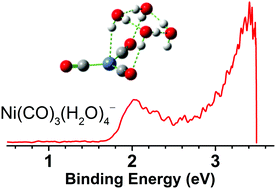
A series of microhydrated nickel carbonyls, Ni(CO)3(H2O)n− (n = 0–4), are prepared via a laser vaporization supersonic cluster source in the gas phase and identified by mass-selected photoelectron velocity-map imaging spectroscopy and quantum chemical calculations. Vertical detachment energies for the n = 1–4 anions are measured from the photoelectron spectra to be 1.429 ± 0.103, 1.698 ± 0.090, 1.887 ± 0.080, and 2.023 ± 0.074 eV, respectively. The C–O stretching vibrational frequencies in the corresponding neutral clusters are determined to be 1968, 1950, 1945, and 1940 cm−1 for n = 1–4, respectively, which are characteristic of terminal CO. It is determined that the hydrogen atom of the first water molecule is bound to the nickel center. Addition of a second water molecule prefers solvation at the carbonyl terminal. Spectroscopy combined with theory suggests that the solvation of nickel tricarbonyl is dominated by a water-ring network. The present findings would have important implications for the fundamental understanding of the multifaceted mechanisms of the multibody interaction of water and carbon monoxide with transition metals.

 Please wait while we load your content...
Please wait while we load your content...