The impact of environment and resonance effects on the site of protonation of aminobenzoic acid derivatives†
Abstract
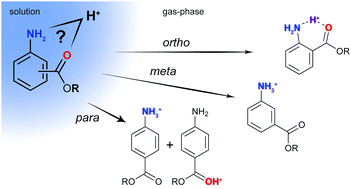
The charge distribution in a molecule is crucial in determining its physical and chemical properties. Aminobenzoic acid derivatives are biologically active small molecules, which have two possible protonation sites: the amine (N-protonation) and the carbonyl oxygen (O-protonation). Here, we employ gas-phase infrared spectroscopy in combination with ion mobility-mass spectrometry and density functional theory calculations to unambiguously determine the preferred protonation sites of p-, m-, and o-isomers of aminobenzoic acids as well as their ethyl esters. The results show that the site of protonation does not only depend on the intrinsic molecular properties such as resonance effects, but also critically on the environment of the molecules. In an aqueous environment, N-protonation is expected to be lowest in energy for all species investigated here. In the gas phase, O-protonation can be preferred, and in those cases, both N- and O-protonated species are observed. To shed light on a possible proton migration pathway, the protonated molecule–solvent complex as well as proton-bound dimers are investigated.


 Please wait while we load your content...
Please wait while we load your content...