The influence of LiH on the rehydrogenation behavior of halide free rare earth (RE) borohydrides (RE = Pr, Er)†
Abstract
Rare earth (RE) metal borohydrides are receiving immense consideration as possible hydrogen storage materials and solid-state Li-ion conductors. In this study, halide free Er(BH4)3 and Pr(BH4)3 have been successfully synthesized for the first time by the combination of mechanochemical milling and/or wet chemistry. Rietveld refinement of Er(BH4)3 confirmed the formation of two different Er(BH4)3 polymorphs: α-Er(BH4)3 with space group Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = 10.76796(5) Å, and β-Er(BH4)3 in Pm
, a = 10.76796(5) Å, and β-Er(BH4)3 in Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m with a = 5.4664(1) Å. A variety of Pr(BH4)3 phases were found after extraction with diethyl ether: α-Pr(BH4)3 in Pa
m with a = 5.4664(1) Å. A variety of Pr(BH4)3 phases were found after extraction with diethyl ether: α-Pr(BH4)3 in Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with a = 11.2465(1) Å, β-Pr(BH4)3 in Pm
with a = 11.2465(1) Å, β-Pr(BH4)3 in Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m with a = 5.716(2) Å and LiPr(BH4)3Cl in I
m with a = 5.716(2) Å and LiPr(BH4)3Cl in I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m, a = 11.5468(3) Å. Almost phase pure α-Pr(BH4)3 in Pa
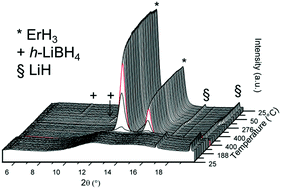
3m, a = 11.5468(3) Å. Almost phase pure α-Pr(BH4)3 in Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with a = 11.2473(2) Å was also synthesized. The thermal decomposition of Er(BH4)3 and Pr(BH4)3 proceeded without the formation of crystalline products. Rehydrogenation, as such, was not successful. However, addition of LiH promoted the rehydrogenation of RE hydride phases and LiBH4 from the decomposed RE(BH4)3 samples.
with a = 11.2473(2) Å was also synthesized. The thermal decomposition of Er(BH4)3 and Pr(BH4)3 proceeded without the formation of crystalline products. Rehydrogenation, as such, was not successful. However, addition of LiH promoted the rehydrogenation of RE hydride phases and LiBH4 from the decomposed RE(BH4)3 samples.


 Please wait while we load your content...
Please wait while we load your content...