Computational prediction and analysis of the 27Al solid-state NMR spectrum of methylaluminoxane (MAO) at variable temperatures and field strengths†
Abstract
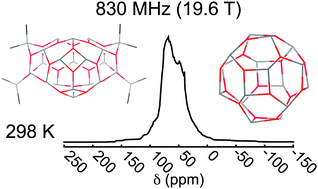
Calculations of NMR shielding tensors and nuclear quadrupole coupling (NQC) tensors at the Kohn–Sham density functional level are used to simulate 27Al magic-angle spinning (MAS) NMR spectra of the important olefin polymerization co-catalyst methylaluminoxane (MAO) at 77, 298, 398, and 498 K and spectrometer magnetic field inductions B ranging from 14.1 to 23.5 T. The calculations utilize the temperature (T) dependent distribution of species present in MAO determined recently by Zurek and coworkers from first-principles theory [Macromolecules, 2014, 47, 8556]. The NMR calculations suggest that variable-T and variable-B NMR measurements are able to quantify the ratio of free versus bound trimethyl-aluminum (TMA) in MAO via characteristic spectral features assigned to 3-coordinate and 4-coordinate Al sites in MAO as well as spectral features arising from free TMA or its dimer. The T-dependent distribution of species causes other characteristic features in the NMR spectra to appear/disappear that can be associated with different aluminum environments such as square vs. hexagonal faces in cage and tubular structures. The simulated spectra at 298 K and 19.6 T are in reasonably good agreement with the experimental solid-state NMR (SSNMR) spectra obtained previously for MAO gel. The promise and limitations of solid-state NMR to unravel the enigma surrounding the structure(s) of MAO are discussed.

 Please wait while we load your content...
Please wait while we load your content...