Observation of a new channel, the production of CH3, in the abstraction reaction of OH radicals with acetaldehyde†
Abstract
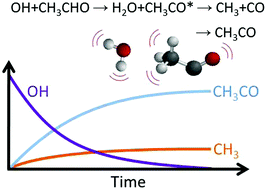
Using laser flash photolysis coupled to photo-ionization time-of-flight mass spectrometry (PIMS), methyl radicals (CH3) have been detected as primary products from the reaction of OH radicals with acetaldehyde (ethanal, CH3CHO) with a yield of ∼15% at 1–2 Torr of helium bath gas. Supporting measurements based on laser induced fluorescence studies of OH recycling in the OH/CH3CHO/O2 system are consistent with the PIMS study. Master equation calculations suggest that the origin of the methyl radicals is from prompt dissociation of chemically activated acetyl products and hence is consistent with previous studies which have shown that abstraction, rather than addition/elimination, is the sole route for the OH + acetaldehyde reaction. However, the observation of a significant methyl product yield suggests that energy partitioning in the reaction is different from the typical early barrier mechanism where reaction exothermicity is channeled preferentially into the newly formed bond. The master equation calculations predict atmospheric yields of methyl radicals of ∼9%. The implications of the observations in atmospheric and combustion chemistry are briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...