Electrostatically enhanced F⋯F interactions through hydrogen bonding, halogen bonding and metal coordination: an ab initio study†
Abstract
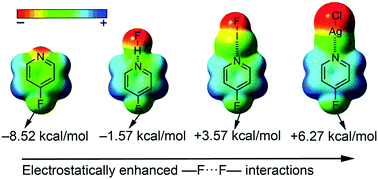
In this manuscript the ability of hydrogen and halogen bonding interactions, as well as metal coordination to enhance F⋯F interactions involving fluorine substituted aromatic rings has been studied at the RI-MP2/def2-TZVPD level of theory. We have used 4-fluoropyridine, 4-fluorobenzonitrile, 3-(4-fluorophenyl)propiolonitrile and their respective meta derivatives as aromatic compounds. In addition, we have used HF and IF as hydrogen and halogen bond donors, respectively, and Ag(I) as the coordination metal. Furthermore, we have also used HF as an electron rich fluorine donor entity, thus establishing F⋯F interactions with the above mentioned aromatic systems. Moreover, a CSD (Cambridge Structural Database) search has been carried out and some interesting examples have been found, highlighting the impact of F⋯F interactions involving aromatic fluorine atoms in solid state chemistry. Finally, cooperativity effects between F⋯F interactions and both hydrogen and halogen bonding interactions have been analyzed and compared. We have also used Bader's theory of “atoms in molecules” to further describe the cooperative effects.


 Please wait while we load your content...
Please wait while we load your content...