The role of the substrate structure in the on-surface synthesis of organometallic and covalent oligophenylene chains†
Abstract
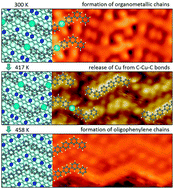
The influences of the substrate structure on the formation of one-dimensional organometallic and covalent oligomers on a Cu(110) surface were studied using scanning tunneling microscopy (STM), X-ray photoemission spectroscopy (XPS), and low energy electron diffraction (LEED) in ultrahigh vacuum (UHV). Vapor deposition of submonolayer 4,4′′-dibromo-meta-terphenyl (DMTP) onto a Cu(110) surface at 300 K leads to scission of C–Br bonds and the formation of organometallic chains (cis/trans and all-trans) connected by C–Cu–C bonds. Larger islands (120 × 120 nm2) of all-trans zigzag organometallic chains as sole products were obtained by the deposition of DMTP onto Cu(110) held at 383 K. The domains are oriented along two directions with an angle of ±13° relative to the [0 0 1] direction due to the two-fold symmetry of the Cu(110) surface lattice. This study reveals at a sub-molecular level that the organometallic chains firstly lose copper atoms and then undergo C–C coupling into oligophenylene chains at a substrate temperature around 417 K. Annealing the large islands of organometallic chains at 458 K results in the formation of completely C–C covalently bonded zigzag oligophenylene chains. The zigzag angle of 125° slightly deviates from the ideal value of 120°. This is attributed to a stretching of the zigzag oligophenylene chains due to substrate template effects.


 Please wait while we load your content...
Please wait while we load your content...