Globular–disorder transition in proteins: a compromise between hydrophobic and electrostatic interactions?†
Abstract
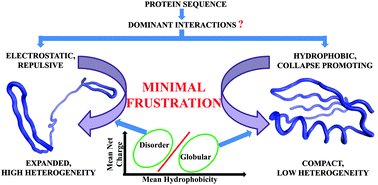
The charge–hydrophobicity correlation of globular and disordered proteins is explored using a generalized self-consistent field theoretical method combined with Monte Carlo simulations. Globular and disordered protein sequences with varied mean net charge and mean hydrophobicity are designed by theory, while Metropolis Monte Carlo generates a suitable ensemble of conformations. Results imply a transition of the dominant interactions between globular and disordered proteins across the charge–hydrophobicity boundary. It is observed that the charge–hydrophobicity boundary actually represents a trade-off between the repulsive and attractive interactions in a protein sequence. The attractive interactions predominate on the globular side of the boundary, while the repulsive interactions prevail on the disordered side. For globular proteins, core forming hydrophobic interactions are dominant leading to a minimally frustrated native conformation. For disordered proteins, the repulsive electrostatic interactions prevail yielding a minimally frustrated region comprising of an expanded, dynamic conformational ensemble. Thus, protein disorder, like protein folding, satisfies the principle of minimal frustration. All results are compared to real globular and disordered proteins. Thus this algorithm may be useful to probe the conformational characteristics of disordered proteins.

 Please wait while we load your content...
Please wait while we load your content...