Understanding abnormal potential behaviors at the 1st charge in Li2S cathode material for rechargeable Li–S batteries†
Abstract
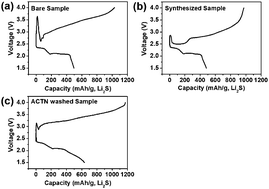
In this study, electrochemical behaviors of Li2S such as a large potential barrier at the beginning of the 1st charging process and a continuous increase in potential to ∼4 V during the rest of this process were understood through X-ray photoelectron spectroscopy measurements and electrochemical evaluations for a full utilization of Li2S. The large potential barrier to the 1st charge in Li2S can be caused by the presence of insulating oxidized products (Li2SO3 or Li2SO4-like structures) on the surface; simple surface etching can remove them and thereby reduce the potential barrier. Even though the potential barrier was substantially reduced, the electrochemical activity of Li2S might not be improved due to the continuous increase in potential. This increase in potential was related to the polarization caused by the Li2S-conversion reaction; the polarization can affect the utilization of Li2S in subsequent cycles. We speculate that the increase in potential is related to the decomposition of oxidized products such as Li2CO3-like or Li2O-like structures on the surface of the Li2S particles. These findings indicate that the full utilization of Li2S can be achieved by controlling their surface characteristics, especially the surface oxidation products.

 Please wait while we load your content...
Please wait while we load your content...