Realizing controllable graphene nucleation by regulating the competition of hydrogen and oxygen during chemical vapor deposition heating
Abstract
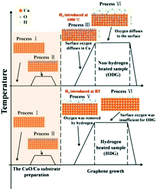
Oxygen can passivate Cu surface active sites when graphene nucleates. Thus, the nucleation density is decreased. The CuO/Cu substrate was chosen for graphene domain synthesis in our study. The results indicate that the CuO/Cu substrate is beneficial for large-scale, single-crystal graphene domain synthesis. Graphene grown on the CuO/Cu substrate exhibits fewer nucleation sites than on Cu foils, suggesting that graphene follows an oxygen-dominating growth. Hydrogen treatment via a heating process could weaken the surface oxygen's role in limiting graphene nucleation under the competition of hydrogen and oxygen and could transfer the synthesis of graphene into a hydrogen-dominating growth. However, the competition only exists during the chemical vapor deposition heating process. For non-hydrogen heated samples, oxygen-dominating growth is experienced even though the samples are annealed in hydrogen for a long time after the heating process. With the temperature increases, the role of hydrogen gradually decreases. The balance of hydrogen and oxygen is adjusted by introducing hydrogen gas at a different heating temperatures. The oxygen concentration on the substrate surface is believed to determine the reactions mechanisms based on the secondary ion mass spectrometry test results. This study provides a new method for the controllable synthesis of graphene nucleation during a heating process.

 Please wait while we load your content...
Please wait while we load your content...