Solvation structure around the Li+ ion in succinonitrile–lithium salt plastic crystalline electrolytes†
Abstract
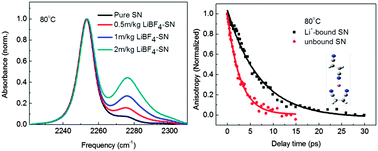
Herein, we discuss the study of solvation dynamics of lithium–succinonitrile (SN) plastic crystalline electrolytes by ultrafast vibrational spectroscopy. The infrared absorption spectra indicated that the CN stretch of the Li+ bound and unbound succinonitrile molecules in a same solution have distinct vibrational frequencies (2276 cm−1vs. 2253 cm−1). The frequency difference allowed us to measure the rotation decay times of solvent molecules bound and unbound to Li+ ion. The Li+ coordination number of the Li+–SN complex was found to be 2 in the plastic crystal phase (22 °C) and 2.5–3 in the liquid phase (80 °C), which is independent of the concentration (from 0.05 mol kg−1 to 2 mol kg−1). The solvation structures along with DFT calculations of the Li+–SN complex have been discussed. In addition, the dissociation percentage of lithium salt was also determined. In 0.5 mol kg−1 LiBF4–SN solutions at 80 °C, 60% ± 10% of the salt dissociates into Li+, which is bound by 2 or 3 solvent molecules. In the 0.5 mol kg−1 LiClO4–SN solutions at 80 °C, the salt dissociation ratio can be up to 90% ± 10%.

 Please wait while we load your content...
Please wait while we load your content...