Influence of electric potential on the apparent viscosity of an ionic liquid: facts and artifacts†
Abstract
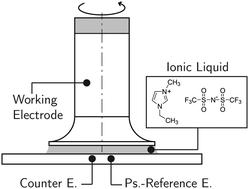
According to recent findings, the steady shear viscosity of the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][Tf2N]) decreases significantly under the influence of electric potential. This implies a causal connection between nanoscale ordering at the electrified interface and a macroscopic change of transport properties. To study this phenomenon in more detail, we reproduced the above-mentioned measurements; however, we find no evidence that the viscosity of [Emim][Tf2N] is a function of electric potential. Additionally, our results show that steady shear measurements can lead to artifacts that, at first glance, may appear to be potential-induced changes in viscosity. We demonstrate that the artifacts result from a sliding electrical contact at the working electrode of the electrochemical cell and we suggest to consider our findings for future viscosity measurements of ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...