Photoisomerization of azobenzenes isolated in cryogenic matrices†
Abstract
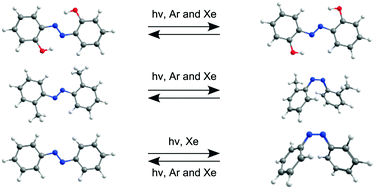
2,2′-Dihydroxyazobenzene (DAB), 2,2′-azotoluene (AT) and azobenzene (AB) were isolated in argon and xenon matrices and their molecular structures and photochemical transformations were characterized by infrared spectroscopy and theoretical calculations. All these compounds can adopt the E and Z isomeric forms around the central CNNC moiety, which can be enriched by several conformational and tautomeric modifications for DAB and AT. A number of DAB and AT isomeric forms were identified for the first time. For DAB, the E azo-enol isomer with two intramolecular six-membered quasi-rings formed via OH⋯N hydrogen bonds was found after deposition. Irradiation with UV light generated a different E azo-enol form with two intramolecular H-bonded five-membered quasi-rings. Phototransformation was shown to be reversible and the forms could be interconverted by irradiation at different wavelengths. The isomerization between these two forms constitutes a direct experimental observation of an E → E isomerization in azobenzene-type molecules. Further irradiation generated a form(s) bearing both OH and NH groups. For AT, two E isomers with the CH3 groups forming five-membered and five/six-membered quasi-rings with the azo group were observed in the as-deposited matrices. Irradiation of AT with UV light generated a Z form that can be converted back to the E form at different irradiation wavelengths. E-AB was deposited in a xenon matrix and both E → Z and Z → E phototransformations were observed (contrary to what was previously reported in an argon matrix where only the Z → E conversion occurred). AB photoisomerization becomes more pronounced at elevated temperatures, thus indicating that the matrix effects responsible for hindering the AB photoisomerization are essentially due to steric restrictions. The different photoisomerization channels observed for these compounds are discussed in terms of a small-amplitude pedal motion.

 Please wait while we load your content...
Please wait while we load your content...