How surface reparation prevents catalytic oxidation of carbon monoxide on atomic gold at defective magnesium oxide surfaces
Abstract
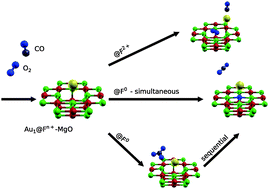
In this contribution, we study using first principles the co-adsorption and catalytic behaviors of CO and O2 on a single gold atom deposited at defective magnesium oxide surfaces. Using cluster models and point charge embedding within a density functional theory framework, we simulate the CO oxidation reaction for Au1 on differently charged oxygen vacancies of MgO(001) to rationalize its experimentally observed lack of catalytic activity. Our results show that: (1) co-adsorption is weakly supported at F0 and F2+ defects but not at F1+ sites, (2) electron redistribution from the F0 vacancy via the Au1 cluster to the adsorbed molecular oxygen weakens the O2 bond, as required for a sustainable catalytic cycle, (3) a metastable carbonate intermediate can form on defects of the F0 type, (4) only a small activation barrier exists for the highly favorable dissociation of CO2 from F0, and (5) the moderate adsorption energy of the gold atom on the F0 defect cannot prevent insertion of molecular oxygen inside the defect. Due to the lack of protection of the color centers, the surface becomes invariably repaired by the surrounding oxygen and the catalytic cycle is irreversibly broken in the first oxidation step.

 Please wait while we load your content...
Please wait while we load your content...