Structure of cobalt protoporphyrin chloride and its dimer, observation and DFT modeling†
Abstract
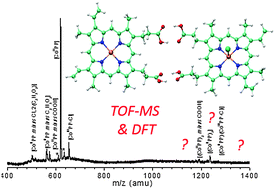
In this article we present a joint study by time-of-flight mass spectroscopy and density functional theory of cobalt protoporphyrin dimer complexes. The main novelty of the experimental part is to reveal the formation of porphyrin dimers that eventually include a chlorine atom. Density functional theory calculations have been performed to shed light on the structural and electronic properties of monomers and dimers that may be formed experimentally. Various geometries of the monomers are analyzed in the two lowest spin states. The electronic structures are examined by means of population analysis relying on the iterative Hirshfeld scheme and the topological analyses of the electron localization function. It is shown that the cobalt ligand bond is purely ionic in the triplet states but shows a noticeable covalent character in the singlet state. Ionization potential of Co-protoporphyrin and binding energies of the chlorine ligand are also reported. Concerning the dimers, several association patterns are investigated for the chlorinated and non-chlorinated complexes. It is found that the structures of the most stable complexes involve four hydrogen bonds between the carboxylic acid moieties of the protoporphyrins. However other association modes are likely to be possible in the experiments.

 Please wait while we load your content...
Please wait while we load your content...