Unveiling the electrochemical mechanisms of Li2Fe(SO4)2 polymorphs by neutron diffraction and density functional theory calculations†
Abstract
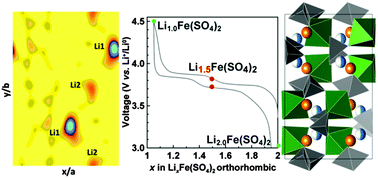
The quest for new sustainable iron-based positive electrode materials for lithium-ion batteries recently led to the discovery of a new family of compounds with the general formula Li2M(SO4)2 with M = transition metal, which presents monoclinic and orthorhombic polymorphs. In terms of electrochemical performances, although both Li2Fe(SO4)2 polymorphs present a similar potential of ∼3.8 V vs. Li+/Li0, the associated electrochemical processes drastically differ in terms of polarization and reaction redox mechanisms. We herein provide an explanation to account for such a behavior. While monoclinic Li2Fe(SO4)2 directly transforms into Li1.0Fe(SO4)2 upon oxidation, the orthorhombic counterpart forms a distinct intermediate Li1.5Fe(SO4)2 phase leading to a two-step delithiation process involving an unequal depopulation of the two Li sites pertaining to the structure as deduced by neutron powder diffraction experiments and confirmed by both density functional theory and Bond Valence Energy Landscape calculations. Moreover, to access band gap information, both polymorphs are studied by UV/Vis spectroscopy. Lastly, the possibility of transforming the monoclinic phase to the orthorhombic phase under pressure is explored.

 Please wait while we load your content...
Please wait while we load your content...