Correlation of the depletion layer with the Helmholtz layer in the anatase TiO2–H2O interface via molecular dynamics simulations
Abstract
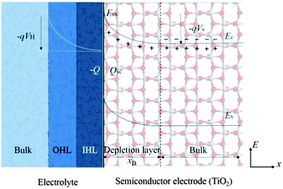
Molecular dynamics simulations have been conducted to study the interaction between anatase TiO2(001), (100), and (101) surfaces and water at room temperature. The dynamic interfacial structure and properties of water on anatase TiO2 surfaces are obtained by analyzing the water density, the diffusion coefficient of water, the surface charge distribution, electric fields and the electrostatic potential distribution. The simulation results have revealed that a highly-ordered water layer structure can be formed near to the anatase TiO2 surface and have also given the Helmholtz layer width and potential drop at the water–TiO2 interface. By correlating the Helmholtz layer with the depletion layer, the depletion layer widths of three surfaces (001), (100), and (101) have been calculated as 474 Å, 237 Å and 99 Å, respectively. The resulting order of the photoelectrochemical activity of the anatase TiO2 surfaces is (001) > (100) > (101), which is consistent with the experimental results. This study may provide a useful correlation of the depletion layer with the Helmholtz layer based on simulations results for the prediction of the behavior and the control of photon-energy conversion devices.

 Please wait while we load your content...
Please wait while we load your content...