Lithium ion solvation by ethylene carbonates in lithium-ion battery electrolytes, revisited by density functional theory with the hybrid solvation model and free energy correction in solution
Abstract
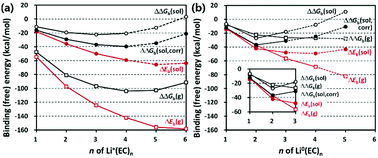
Complex formation between lithium (Li+) ions and electrolyte molecules would affect the ionic conductivity through the electrolyte in lithium-ion batteries (LIBs). We hence revisit the solvation number of Li+ in the most commonly used ethylene carbonate (EC) electrolyte. The solvation number n of Li+(EC)n in the first solvation shell of Li+ is estimated on the basis of the free energy calculated by the density functional theory combined with a hybrid solvation model where the explicit solvation shell of Li+ is immersed in a free volume of an implicit bulk solvent. This new hybrid solvation (implicit and explicit) model predicts the most probable solvation number (n = 4) and solvation free energy (−91.3 kcal mol−1) of Li+ in a good agreement with those predicted by calculations employing simpler solvation models (either implicit or explicit). The desolvation (n = 2) of Li0(EC)n upon reduction near anodes is also well described with this new hybrid model.

 Please wait while we load your content...
Please wait while we load your content...