Partial oxidation of step-bound water leads to anomalous pH effects on metal electrode step-edges
Abstract
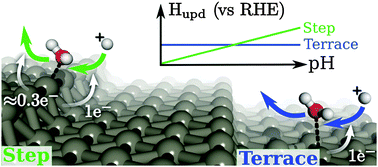
The design of better heterogeneous catalysts for applications such as fuel cells and electrolyzers requires a mechanistic understanding of electrocatalytic reactions and the dependence of their activity on operating conditions such as pH. A satisfactory explanation for the unexpected pH dependence of electrochemical properties of platinum surfaces has so far remained elusive, with previous explanations resorting to complex co-adsorption of multiple species and resulting in limited predictive power. This knowledge gap suggests that the fundamental properties of these catalysts are not yet understood, limiting systematic improvement. Here, we analyze the change in charge and free energies upon adsorption using density-functional theory (DFT) to establish that water adsorbs on platinum step edges across a wide voltage range, including the double-layer region, with a loss of approximately 0.2 electrons upon adsorption. We show how this as-yet unreported change in net surface charge due to this water explains the anomalous pH variations of the hydrogen underpotential deposition (Hupd) and the potentials of zero total charge (PZTC) observed in published experimental data. This partial oxidation of water is not limited to platinum metal step edges, and we report the charge of the water on metal step edges of commonly used catalytic metals, including copper, silver, iridium, and palladium, illustrating that this partial oxidation of water broadly influences the reactivity of metal electrodes.


 Please wait while we load your content...
Please wait while we load your content...