Structural properties of geminal dicationic ionic liquid/water mixtures: a theoretical and experimental insight†
Abstract
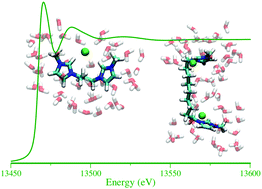
The structural behavior of geminal dicationic ionic liquid 1,n-bis[3-methylimidazolium-1-yl] alkane bromide ([Cn(mim)2]Br2)/water mixtures has been studied using extended X-ray absorption fine structure (EXAFS) spectroscopy in combination with molecular dynamics (MD) simulations. The properties of the mixtures are investigated as a function of both water concentration and alkyl-bridge chain length. The very good agreement between the EXAFS experimental data and the theoretical curves calculated from the MD structural results has proven the validity of the theoretical framework used for all of the investigated systems. In all the solutions the water molecules are preferentially coordinated with the Br− ion, even if a complex network of interactions among dications, anions and water molecules takes place. The local molecular arrangement around the bromide ion is found to change with increasing water content, as more and more water molecules are accomodated in the Br− first coordination shell. Moreover, with the decrease of the alkyl-bridge chain length, the interactions between dications and anions increase, with Br− forming a bridge between the two imidazolium rings of the same dication. On the other hand, in [Cn(mim)2]Br2/water mixtures with long alkyl-bridge chains peculiar internal arrangements of the dications are found, leading to different structural features of geminal dicationic ionic liquids as compared to their monocationic counterparts.

 Please wait while we load your content...
Please wait while we load your content...