New insight into probe-location dependent polarity and hydration at lipid/water interfaces: comparison between gel- and fluid-phases of lipid bilayers†
Abstract
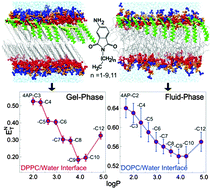
Environment polarity and hydration at lipid/water interfaces play important roles in membrane biology, which are investigated here using a new homologous series of 4-aminophthalimide-based fluorescent molecules (4AP-Cn; n = 2–10, 12) having different lipophilicities (octanol/water partition coefficient – log P). We show that 4AP-Cn molecules probe a peculiar stepwise polarity (ENT) profile at the lipid/water interface of the gel-phase (Lβ′) DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) bilayer at room temperature, which was not anticipated in earlier studies. However, the same molecules probe only a subtle but continuous polarity change at the interface of water and the fluid-phase (Lα) DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) bilayer at room temperature. Fluorescence quenching experiments indicate that solutes with different log P values adsorb at different depths across DPPC/water and DOPC/water interfaces, which correlate with the polarity profiles observed at the interfaces. Molecular dynamics simulations performed on eight probe–lipid systems (four in each of the DPPC and DOPC bilayers – a total run of 2.6 μs) support experimental results, providing further information on the relative position and angle distributions as well as hydration of probes at the interfaces. Simulation results indicate that besides positions, probe orientations also play an important role in defining the local dielectric environment by controlling the probes' exposure to water at the interfaces especially of the gel-phase DPPC bilayer. The results suggest that 4AP-Cn probes are well suited for studying solvation properties at lipid/water interfaces of gel- and fluid-phases simultaneously.

 Please wait while we load your content...
Please wait while we load your content...