Conformation-specific spectroscopy of capped glutamine-containing peptides: role of a single glutamine residue on peptide backbone preferences†
Abstract
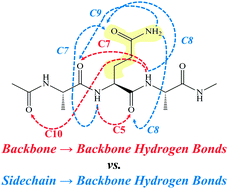
The conformational preferences of a series of short, aromatic-capped, glutamine-containing peptides have been studied under jet-cooled conditions in the gas phase. This work seeks a bottom-up understanding of the role played by glutamine residues in directing peptide structures that lead to neurodegenerative diseases. Resonant ion-dip infrared (RIDIR) spectroscopy is used to record single-conformation infrared spectra in the NH stretch, amide I and amide II regions. Comparison of the experimental spectra with the predictions of calculations carried out at the DFT M05-2X/6-31+G(d) level of theory lead to firm assignments for the H-bonding architectures of a total of eight conformers of four molecules, including three in Z-Gln-OH, one in Z-Gln-NHMe, three in Ac-Gln-NHBn, and one in Ac-Ala-Gln-NHBn. The Gln side chain engages actively in forming H-bonds with nearest-neighbor amide groups, forming C8 H-bonds to the C-terminal side, C9 H-bonds to the N-terminal side, and an amide-stacked geometry, all with an extended (C5) peptide backbone about the Gln residue. The Gln side chain also stabilizes an inverse γ-turn in the peptide backbone by forming a pair of H-bonds that bridge the γ-turn and stabilize it. Finally, the entire conformer population of Ac-Ala-Gln-NHBn is funneled into a single structure that incorporates the peptide backbone in a type I β-turn, stabilized by the Gln side chain forming a C7 H-bond to the central amide group in the β-turn not otherwise involved in a hydrogen bond. This β-turn backbone structure is nearly identical to that observed in a series of X-(AQ)-Y β-turns in the protein data bank, demonstrating that the gas-phase structure is robust to perturbations imposed by the crystalline protein environment.

 Please wait while we load your content...
Please wait while we load your content...