Synthesis, physical properties and application of the zero-valent iron/titanium dioxide heterocomposite having high activity for the sustainable photocatalytic removal of hexavalent chromium in water†
Abstract
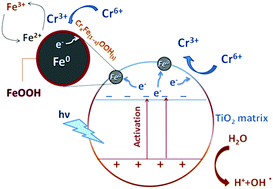
A magnetic photocatalytic material composed of nanoscale zero-valent iron (nZVI) homogeneously distributed over a mesoporous nanocrystalline TiO2 matrix has been prepared by a multistage chemical process, including sol–gel technique, wet impregnation, and chemical reduction. X-ray powder diffraction and Raman spectroscopy were used for the structural and chemical characterization of the magnetic photocatalyst, while bulk magnetization measurements and scanning/transmission electron microscopy were employed to determine the physical and textural properties of the photocatalyst. The synthesized nZVI@TiO2 photocatalyst shows very high efficiency in the removal of hexavalent chromium, Cr(VI), from water. The degradation rate follows a pseudo-first-order kinetic model. Most importantly, the remarkable efficiency of the photocatalyst is found to be due to the synergistic contributions of both counterparts, nZVI and TiO2, as validated by comparative experiments with neat TiO2 and nZVI@TiO2 under UV-C irradiation and without irradiation. New insights into the mechanism of synergistic degradation of chromium(VI) and suppressed oxidation of nZVI particles in the composite material are proposed and therein discussed.

 Please wait while we load your content...
Please wait while we load your content...