A blue 4′,7-diaminoflavylium cation showing an extended pH range stability†
Abstract
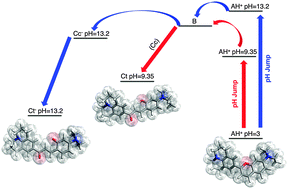
The introduction of two amine substituents in 4′ and 7 positions, leads to the formation of a blue flavylium cation, 7-(N,N′-diethylamino)-2-(9-julolidine)-1-benzopyrilium, which is extremely stable across a wide acidic pH range. The kinetic and thermodynamic constants of the multistate system have been calculated by studying the relaxation kinetics after equilibrium perturbation by addition of base (direct pH jumps) or acid (reverse pH jumps). Except for the cis-chalcone, which is an elusive species, the relative energy levels of the other species could be calculated and a global energy level diagram constructed. The diagram explains that the stability of the diamino compound is due to the high energy level of the hemiketal species, which is difficult to access in acidic medium.

 Please wait while we load your content...
Please wait while we load your content...