Ni on the CeO2(110) and (100) surfaces: adsorption vs. substitution effects on the electronic and geometric structures and oxygen vacancies†
Abstract
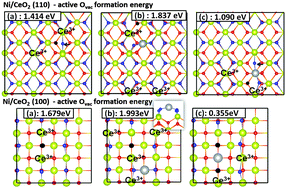
We report density functional theory (DFT) calculations of the interactions of both Ni adsorbate and substitutional dopant with the ceria (110) and (100) surfaces to explain the origin of the activity of Ni/ceria catalysts. Our results indicate that the Ni adatom on the (110) surface prefers to adsorb on a two-fold bridge site over a hollow site up to 0.25 ML coverage, and the most stable position of a Ni adsorbate on the (100) surface was found to be the bridge site where the Ni atom is coordinated to two surface O atoms. The Ni+ oxidation state for the Ni adatom on the (110) surface was found to be more favorable than the Ni2+ state on the two-fold bridge site while on the (100) surface, a Ni adatom prefers its Ni2+ oxidation state over the Ni+ oxidation state. With increasing coverage, the binding energy of a Ni adatom on the (110) surface was found to decrease from −0.45 eV at 0.083 ML coverage to −0.32 eV at 0.25 ML coverage. Oxidation of the Ni adatom to Ni+ reduces one Ce4+ ion on the ceria surface to Ce3+ which preferred to be located next to the Ni+ ion in the nearest neighbor location. The Ce3+ ions on the (100) surface also prefer to stay in the vicinity of the adsorbed Ni atom, while they prefer to be located away from the Ni adatom on the (111) surface. No reduction of Ce4+ ions was observed upon substitution of Ce atoms by Ni atoms. Two Ni substituents preferred to be distributed on adjacent metal ion sites on the (110) surface. Ni adsorbate and substituent on the (110) surface were both found to induce significant structural distortions. In comparison to the pure ceria (110) and (100) surfaces, we show that a Ni adsorbate increases the energy required to create an oxygen vacancy while a Ni dopant reduces it. While multiple dopants on the (110) surface do reduce the vacancy formation energy, the degree of reduction is smaller compared to a single dopant indicating the presence of an optimum level of doping to obtain enhanced catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...