Proton coupled electron transfer from the excited state of a ruthenium(ii) pyridylimidazole complex†
Abstract
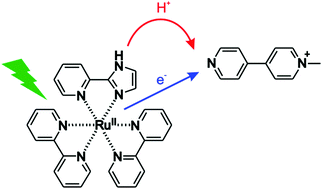
Proton coupled electron transfer (PCET) from the excited state of [Ru(bpy)2pyimH]2+ (bpy = 2,2′-bipyridine; pyimH = 2-(2′-pyridyl)imidazole) to N-methyl-4,4′-bipyridinium (monoquat, MQ+) was studied. While this complex has been investigated previously, our study is the first to show that the formal bond dissociation free energy (BDFE) of the imidazole-N–H bond decreases from (91 ± 1) kcal mol−1 in the electronic ground state to (43 ± 5) kcal mol−1 in the lowest-energetic 3MLCT excited state. This makes the [Ru(bpy)2pyimH]2+ complex a very strong (formal) hydrogen atom donor even when compared to metal hydride complexes, and this is interesting for light-driven (formal) hydrogen atom transfer (HAT) reactions with a variety of different substrates. Mechanistically, formal HAT between 3MLCT excited [Ru(bpy)2pyimH]2+ and monoquat in buffered 1 : 1 (v : v) CH3CN/H2O was found to occur via a sequence of reaction steps involving electron transfer from Ru(II) to MQ+ coupled to release of the N–H proton to buffer base, followed by protonation of reduced MQ+ by buffer acid. Our study is relevant in the larger contexts of photoredox catalysis and light-to-chemical energy conversion.

 Please wait while we load your content...
Please wait while we load your content...