Phenomenological thermodynamics and the structure formation mechanism of the CuTi2S4 rhombohedral phase
Abstract
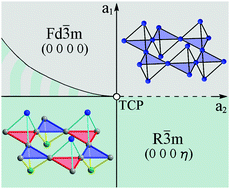
The theory of structural phase transition in CuTi2S4 is proposed. The symmetry of order parameters, thermodynamics and the mechanism of the atomic structure formation of the rhombohedral Cu–Ti–thiospinel have been studied. The critical order parameter inducing the phase transition has been found. Within the Landau theory of phase transitions, it is shown that the phase state may change from the high-symmetry cubic disordered Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase to the low-symmetry ordered rhombohedral R
m phase to the low-symmetry ordered rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase as a result of phase transition of the first order close to the second order. It is shown that the rhombohedral structure of CuTi2S4 is formed as a result of the displacements of all types of atoms and the ordering of Cu-atoms (1 : 1 order type in tetrahedral spinel sites), Ti-atoms (1 : 1 : 6 order type in octahedral spinel sites), and S-atoms (1 : 1 : 3 : 3 order type). The Cu- and Ti-atoms form metal nanoclusters which are named a “bunch” of dimers. The “bunch” of dimers in CuTi2S4 is a new type of self-organization of atoms in frustrated spinel-like structures. It is shown that Ti-atoms also form other types of metal nanoclusters: trimers and tetrahedra.
m phase as a result of phase transition of the first order close to the second order. It is shown that the rhombohedral structure of CuTi2S4 is formed as a result of the displacements of all types of atoms and the ordering of Cu-atoms (1 : 1 order type in tetrahedral spinel sites), Ti-atoms (1 : 1 : 6 order type in octahedral spinel sites), and S-atoms (1 : 1 : 3 : 3 order type). The Cu- and Ti-atoms form metal nanoclusters which are named a “bunch” of dimers. The “bunch” of dimers in CuTi2S4 is a new type of self-organization of atoms in frustrated spinel-like structures. It is shown that Ti-atoms also form other types of metal nanoclusters: trimers and tetrahedra.

 Please wait while we load your content...
Please wait while we load your content...