Underlying mechanisms of the synergistic role of Li2MnO3 and LiNi1/3Co1/3Mn1/3O2 in high-Mn, Li-rich oxides†
Abstract
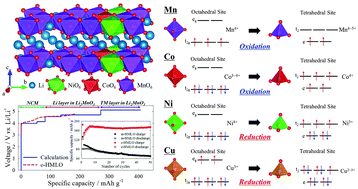
For large-scale energy storage applications requiring high energy density, the development of Li-rich oxides with enhanced cyclic stabilities during high-voltage operations and large specific capacities is required. In this regard, high-Mn, Li-rich oxides (HMLOs; xLi2MnO3 (1 − x)LiNi1/3Co1/3Mn1/3O2 at x > 0.5) warrant an in-depth study because of their good cyclic performance at high operating voltages and potentially large specific capacities. Here, to understand the synergistic effects and enhanced cyclic stability of HMLOs, mechanically blended HMLO (m-HMLO) and chemically bonded HMLO (c-HMLO) were prepared and investigated. c-HMLO exhibits relatively high reaction voltages, large specific capacities, and enhanced cyclic stabilities (∼99%) at a high operating voltage (∼4.8 V vs. Li/Li+) compared with m-HMLO. First-principles calculations with electronic structure analysis were performed using an atomic model developed by Rietveld refinement using as-synthesised c-HMLO. The redox mechanisms of Ni, Co, and Mn ions were determined via the partial density of states of the ground states predicted using the cluster expansion method, which elucidates that LiNi1/3Co1/3Mn1/3O2 stabilises the transition metal (TM) layer of Li2MnO3 and separates Li delithiation potentials in Li2MnO3 in the HMLO. Kinetic analyses including electronic structures revealed that the interlayer migration of TMs from the TM layer to the Li layer depends on the crystal field stabilisation. Thus, TMs with reduced character in the tetrahedral sites than the octahedral sites owing to the effects of crystal field stabilisation, such as Ni ions, in HMLOs would face a higher interlayer migration barrier, impeding phase transformation into spinel phases. Furthermore, Cu ions could constitute a doping source for HMLOs to improve the material’s cyclic stability through this mechanism. These characteristics may be widely applied to explain experimental phenomena and improve the properties of cathode materials for Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...