Computer simulations of 3C-SiC under hydrostatic and non-hydrostatic stresses
Abstract
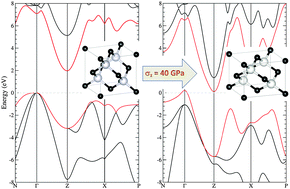
The response of 3C-SiC to hydrostatic pressure and to several uni- and bi-axial stress conditions is thoroughly investigated using first principles calculations. A topological interpretation of the chemical bonding reveals that the so-called non-covalent interactions enhance only at high pressure while the nature of the covalent Si–C bonding network keeps essentially with the same pattern. The calculated low compressibility agrees well with experimental values and is in concordance with the high structural stability of this polymorph under hydrostatic pressure. Under uniaxial [001] stress, the c/a ratio shows a noticeable drop inducing a closure of the band gap and the emergence of a metallic state around 40 GPa. This behavior correlates with a plateau of the electron localization function exhibiting a roughly constant and non-negligible value surrounding CSi4 and SiC4 covalent bonded units.

 Please wait while we load your content...
Please wait while we load your content...