Globally stable structures of LixZn (x = 1–4) compounds at high pressures
Abstract
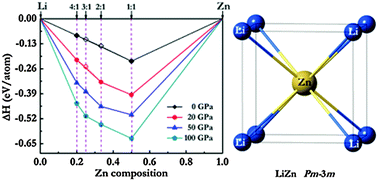
Pressure can change the properties of atoms and bonding patterns, leading to the synthesis of novel compounds with interesting properties. The intermetallic lithium–zinc (Li–Zn) compounds have attracted increasing attention because of their fascinating mechanical properties and widespread applications in rechargeable Li-ion batteries. Using the effective CALYPSO searching method in combination with first-principles calculations, we theoretically investigated the LixZn (x = 1–4) compounds at pressures of 0 to 100 GPa. We found several stable structures with a variety of stoichiometries and the phase diagram on the Li-rich side under high pressure. The electronic structures of these compounds reveal transferred charges from lithium to zinc mainly fill Zn 4p states and compounds with negatively charged Zn atoms are dramatic. We also calculated the elastic constants to discuss their mechanical properties. Our results enrich the crystal structures of the Li–Zn system and provide a further understanding of structural features and their properties.

 Please wait while we load your content...
Please wait while we load your content...