Al atom on MoO3(010) surface: adsorption and penetration using density functional theory†
Abstract
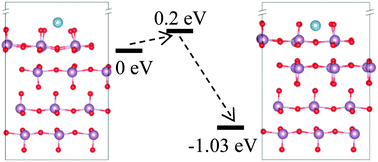
Interfacial issues, such as the interfacial structure and the interdiffusion of atoms at the interface, are fundamental to the understanding of the ignition and reaction mechanisms of nanothermites. This study employs first-principle density functional theory to model Al/MoO3 by placing an Al adatom onto a unit cell of a MoO3(010) slab, and to probe the initiation of interfacial interactions of Al/MoO3 nanothermite by tracking the adsorption and subsurface-penetration of the Al adatom. The calculations show that the Al adatom can spontaneously go through the topmost atomic plane (TAP) of MoO3(010) and reach the 4-fold hollow adsorption-site located below the TAP, with this subsurface adsorption configuration being the most preferred one among all plausible adsorption configurations. Two other plausible configurations place the Al adatom at two bridge sites located above the TAP of MoO3(010) but the Al adatom can easily penetrate below this TAP to a relatively more stable adsorption configuration, with a small energy barrier of merely 0.2 eV. The evidence of subsurface penetration of Al implies that Al/MoO3 likely has an interface with intermixing of Al, Mo and O atoms. These results provide new insights on the interfacial interactions of Al/MoO3 and the ignition/combustion mechanisms of Al/MoO3 nanothermites.

 Please wait while we load your content...
Please wait while we load your content...