Tuning protein–protein interactions using cosolvents: specific effects of ionic and non-ionic additives on protein phase behavior†
Abstract
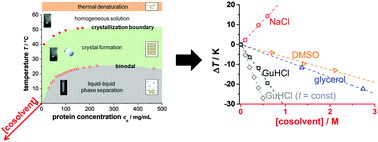
Cosolvents are routinely used to modulate the (thermal) stability of proteins and, hence, their interactions with proteins have been studied intensely. However, less is known about their specific effects on protein–protein interactions, which we characterize in terms of the protein phase behavior. We analyze the phase behavior of lysozyme solutions in the presence of sodium chloride (NaCl), guanidine hydrochloride (GuHCl), glycerol, and dimethyl sulfoxide (DMSO). We experimentally determined the crystallization boundary (XB) and, in combination with data on the cloud-point temperatures (CPTs), the crystallization gap. In agreement with other studies, our data indicate that the additives might affect the protein phase behavior through electrostatic screening and additive-specific contributions. At high salt concentrations, where electrostatic interactions are screened, both the CPT and the XB are found to be linear functions of the additive concentration. Their slopes quantify the additive-specific changes of the phase behavior and thus of the protein–protein interactions. While the specific effect of NaCl is to induce attractions between proteins, DMSO, glycerol and GuHCl (with increasing strength) weaken attractions and/or induce repulsions. Except for DMSO, changes of the CPT are stronger than those of the XB. Furthermore, the crystallization gap widens in the case of GuHCl and glycerol and narrows in the case of NaCl. We relate these changes to colloidal interaction models, namely square-well and patchy interactions.

 Please wait while we load your content...
Please wait while we load your content...