Dissolved chloride markedly changes the nanostructure of the protic ionic liquids propylammonium and ethanolammonium nitrate†
Abstract
The bulk nanostructure of 15 mol% propylammonium chloride (PACl) dissolved in propylammonium nitrate (PAN) and 15 mol% ethanolammonium chloride (EtACl) in ethanolammonium nitrate (EtAN) has been determined using neutron diffraction with empirical potential structure refinement fits. For both the PAN:PACl and EtAN:EtACl mixtures, data for three different scattering contrasts were simultaneously fit, and the structures determined and compared to that of the pure ionic liquids. Strong electrostatic interactions between chloride and cation charged groups, as well as the alcohol moiety of EtAN, lead to marked changes in local ion packing that alter the liquid structure. In PAN, the addition of chloride modifies but does not significantly disrupt the bicontinuous amphiphilic nanostructure of the IL. Tight packing of ammonium groups around chloride favours a gauche conformer for the cation which shrinks the apolar domains and brings the terminal methyls nearer the polar domains. The weakly-clustered nanostructure of EtAN, a consequence of the terminal hydroxyl, is overwhelmed by strong chloride–cation interactions. Ethanolammonium binds tightly to chloride in a monodentate fashion via either its alcohol or ammonium charge centre, or through both in a bidentate arrangement by adopting a gauche or eclipsed conformer.
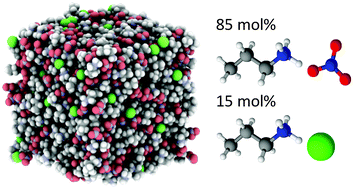
- This article is part of the themed collection: Neutron Scattering in Catalysis and Energy Materials

 Please wait while we load your content...
Please wait while we load your content...