Evaluating bulk Nb2O2F3 for Li-battery electrode applications†
Abstract
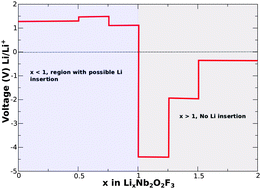
This investigation has the primary objective of elucidating the lithium intercalation process in the crystal structure of a new niobium oxyfluoride compound Nb2O2F3. The framework of the density functional theory was applied in a generalized gradient approximation together with the hybrid functional method. It is revealed that lithium atoms intercalate in this material in a maximum concentration of one Li atom per formula unit forming LiNb2O2F3. Moreover, octahedral positions in between the layers of Nb–O–F appear as the Li preferred occupancy resulting in a structural volume expansion of only 5%. Electronic structure evolution with the insertion of lithium displays a transformation from semi-conductor to metal when half of the lithium atoms are added. This transformation occurs due to a symmetry break induced by the transition from the +8 to +7 oxidation state of half of the Nb2 dimers. Then, after full lithiation the symmetry is recovered and the material becomes a semiconductor again with a band gap amounting to 1 eV. The evaluated average deintercalation potential reaches 1.29 V vs. Li/Li+ with activation energy for lithium ion migration of 0.79 eV. The computed low potential of the redox reaction Nb28+ to Nb27+ includes niobium oxyfluoride in the map of possible materials for the anode application of Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...