Aggregation of α-crystallins in kynurenic acid-sensitized UVA photolysis under anaerobic conditions†
Abstract
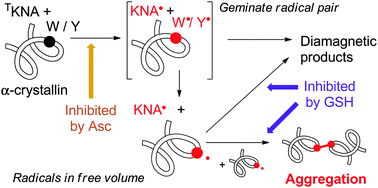
The reactions of photoexcited kynurenic acid (KNA) with bovine α-crystallins under anaerobic conditions proceed via the electron transfer from tryptophan (Trp) and tyrosine (Tyr) residues to the triplet KNA molecules. The subsequent radical reactions lead to the protein aggregation and insolubilization. The absorption of the photolyzed proteins at 335 nm as well as their total fluorescence significantly increases, while the tryptophan-related fluorescence decreases. It has been established that the alterations of the protein optical properties are related to the modifications of Trp residues. Intrinsic lens antioxidants ascorbate (Asc) and glutathione (GSH) that are present in the human lens at the millimolar level effectively block the formation of the observed light-induced protein modifications. The protective effect of Asc was attributed to its ability to quench highly reactive triplet states, while the role of GSH, most likely, corresponds to the reduction of photochemically formed radicals into a diamagnetic state. The results obtained disclose the possible mechanism of UVA-induced modifications of the lens crystallins, leading to the formation of cataract, and the role of major lens antioxidants Asc and GSH in the protection of the lens proteins.

 Please wait while we load your content...
Please wait while we load your content...