Design of new disulfide-based organic compounds for the improvement of self-healing materials
Abstract
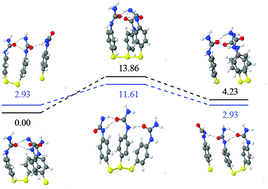
Self-healing materials are a very promising kind of materials due to their capacity to repair themselves. Among others, diphenyl disulfide-based compounds (Ph2S2) appear to be among the best candidates to develop materials with optimum self-healing properties. However, few is known regarding both the reaction mechanism and the electronic structure that make possible such properties. In this vein, theoretical approaches are of great interest. In this work, we have carried out theoretical calculations on a wide set of different disulfide compounds, both aromatic and aliphatic, in order to elucidate the prevalent reaction mechanism and the necessary electronic conditions needed for improved self-healing properties. Two competitive mechanisms were considered, namely, the metathesis and the radical-mediated mechanism. According to our calculations, the radical-mediated mechanism is the responsible for this process. The formation of sulfenyl radicals strongly depends on the S–S bond strength, which can be modulated chemically by the use of proper derivatives. At this point, amino derivatives appear to be the most promising ones. In addition to the S–S bond strength, hydrogen bonding between disulfide chains seems to be relevant to favour the contact among disulfide units. This is crucial for the reaction to take place. The calculated hydrogen bonding energies are of the same order of magnitude as the S–S bond energies. Finally, reaction barriers have been analysed for some promising candidates. Two reaction mechanisms were compared, namely, the [2+2] metathesis reaction mechanism and the [2+1] radical-mediated mechanism. No computational evidence for the existence of any transition state for the metathesis mechanism was found, which indicates that the radical-mediated mechanism is the one responsible in the self-healing process of these materials. Interestingly, the calculated reaction barriers are around 10 kcal mol−1 regardless the substituent employed. All these results suggest that the radical formation and the structural role of the hydrogen bonding prevale over kinetics. Having this in mind, as a conclusion, some new compounds are proposed for the design of future self-healing materials with improved features.

 Please wait while we load your content...
Please wait while we load your content...