In situ characterization of the decomposition behavior of Mg(BH4)2 by X-ray Raman scattering spectroscopy
Abstract
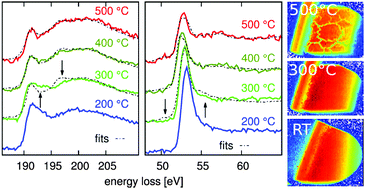
We present an in situ study of the thermal decomposition of Mg(BH4)2 in a hydrogen atmosphere of up to 4 bar and up to 500 °C using X-ray Raman scattering spectroscopy at the boron K-edge and the magnesium L2,3-edges. The combination of the fingerprinting analysis of both edges yields detailed quantitative information on the reaction products during decomposition, an issue of crucial importance in determining whether Mg(BH4)2 can be used as a next-generation hydrogen storage material. This work reveals the formation of reaction intermediate(s) at 300 °C, accompanied by a significant hydrogen release without the occurrence of stable boron compounds such as amorphous boron or MgB12H12. At temperatures between 300 °C and 400 °C, further hydrogen release proceeds via the formation of higher boranes and crystalline MgH2. Above 400 °C, decomposition into the constituting elements takes place. Therefore, at moderate temperatures, Mg(BH4)2 is shown to be a promising high-density hydrogen storage material with great potential for reversible energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...