Orientation of polar molecules near charged protein interfaces†
Abstract
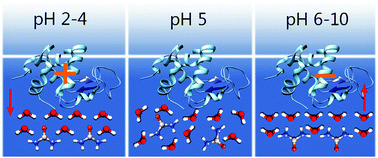
We study the orientation of water and urea molecules and protein amide vibrations at aqueous α-lactalbumin and α-lactalbumin/urea interfaces using heterodyne-detected vibrational sum frequency generation. We vary the net charge of the protein by changing the pH. We find that the orientation of the water and urea molecules closely follows the net charge of the protein at the surface of the solution. In contrast, the net orientation of the amide groups of the backbone of the protein is independent of pH. We discuss the implications of these results for the mechanism by which urea denatures proteins.

 Please wait while we load your content...
Please wait while we load your content...