Probing the early stages of solvation of cis-pinate dianions by water, acetonitrile, and methanol: a photoelectron spectroscopy and theoretical study†
Abstract
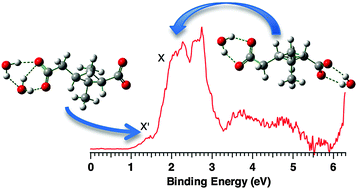
cis-Pinic acid is one of the most important oxidation products of α-pinene – a key monoterpene compound in biogenic emission processes. Molecular level understanding of its interaction with water in cluster formation is an important and necessary prerequisite for ascertaining its role in the aerosol formation processes. In this work, we studied the structures and energetics of the solvated clusters of cis-pinate (cis-PA2−), the doubly deprotonated dicarboxylate of cis-pinic acid, with H2O, CH3OH, and CH3CN by negative ion photoelectron spectroscopy and ab initio theoretical calculations. We found that cis-PA2− prefers being solvated alternately on the two –CO2− groups with increase of solvent coverage, a well-known solvation pattern that has been observed in microhydrated linear dicarboxylate dianion (DCn2−) clusters. Experiments and calculations further reveal an intriguing feature for the existence of the asymmetric type isomers for cis-PA2−(H2O)2 and cis-PA2−(CH3OH)2, in which both solvent molecules interact with only one of the –CO2− groups, a phenomenon that has not been observed in DCn2− water clusters and exhibits that the subtle effect of the rigid four-membered carbon ring brought on the cis-PA2− solvation. The dominant interactions between cis-PA2− and solvent molecules form bidentate O−⋯H–O H-bonds for H2O, O−⋯H–O and O−⋯H–C H-bonds for CH3OH, and tridentate O−⋯H–C H-bonds for CH3CN. The formation of inter-solvent H-bonds between H2O and CH3CN is found to be favorable in mixed solvent clusters, different from that between H2O and CH3OH. These findings have important implications for understanding the mechanism of cluster growth and the formation of atmospheric organic aerosols, as well as for rationalizing the nature of structure–function relationship of proteins containing carboxylate groups in various solvent environments.

 Please wait while we load your content...
Please wait while we load your content...