Structural, electronic, magnetic and chemical properties of B-, C- and N-doped MgO(001) surfaces
Abstract
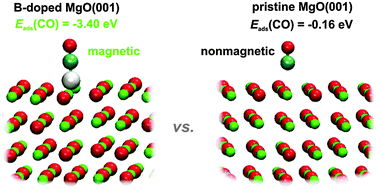
Doping of simple oxide materials can give rise to new exciting physical and chemical properties and open new perspectives for a variety of possible applications. Here we use density functional theory calculations to investigate the B-, C- and N-doped MgO(001) surfaces. We have found that the investigated dopants induce magnetization of the system amounting to 3, 2 and 1 μB for B, C and N, respectively. The dopants are found to be in the X2− state and tend to segregate to the surface. These impurity sites also present the centers of altered chemical reactivity. We probe the chemisorption properties of the doped MgO(001) surfaces with the CO molecule and atomic O. The adsorption of CO is much stronger on B- and C-doped MgO(001) compared to pure MgO(001) as the impurity sites serve as potent electron donors. The situation is similar to the case of atomic oxygen, for which we find the adsorption energy of −8.78 eV on B-doped MgO(001). The surface reactivity changes locally around the dopant atom, which is mainly restricted to its first coordination shell. The presented results suggest doped MgO as a versatile multifunctional material with possible use as an adsorbent or a catalyst.

 Please wait while we load your content...
Please wait while we load your content...