Hydrogen bonding inside and outside carbon nanotubes: HF dimer as a case study†
Abstract
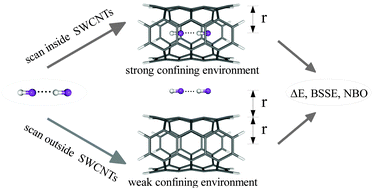
In this theoretical work we analyze the noncovalent interactions of molecular complexes formed between the hydrogen bonded HF dimer and single-walled carbon nanotubes (SWCNTs) of different diameters. In particular, the interaction energies of: (i) spatially confined hydrogen fluoride molecules and (ii) HF dimer and the exterior or interior of SWCNTs are investigated. The computations are carried out in a supermolecular manner using the M06-2X exchange–correlation functional. In order to establish the influence of mutual orientation of the hydrogen fluoride dimer and molecular carbon cages on the analyzed energetic parameters energy scans are performed. Furthermore, changes in the charge distribution of the investigated endo- and exohedral complexes are studied employing the Natural Bond Orbital analysis. Among others, the position of the HF dimer with respect to the carbon cages proves to have a significant influence on the analyzed quantities. The results of our study also indicate that the HF dimer interacts stronger with the interior rather than the exterior of SWCNTs. Moreover, a substantial enhancement of the basis set superposition error is disclosed.

 Please wait while we load your content...
Please wait while we load your content...